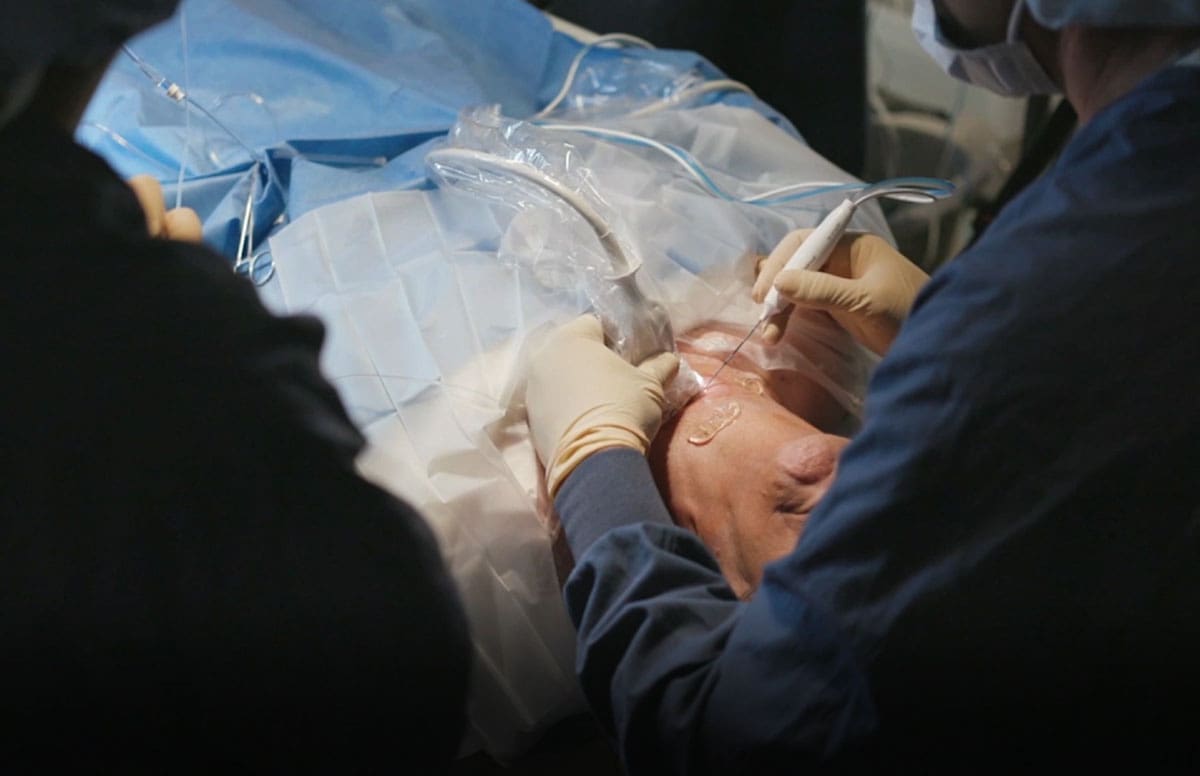
Summary: The blog discusses the expansion and future of thyroid RFA technology in America, highlighting its increased adoption and continuous innovation driven by clinician feedback and academic research.
- Innovation and Market Dominance: STARmed introduced thyroid RFA for thyroid in 2002, leading to widespread clinical use and continuous improvements in RFA electrode technology.
- Potential for Widespread Use: Factors like cost-effectiveness, reduced recovery time, and minimal post-procedure care boost RFA’s adoption prospects.
- Expanding Clinical Training: Enhanced training opportunities, both online and in-person, are expected to boost clinician proficiency in thyroid RFA.
- Patient Awareness: Efforts to educate patients about RFA as a viable treatment option are crucial for its adoption.
- Future Directions: Institutions like Columbia University and Tulane University are conducting research to advance the safety and effectiveness of thyroid RFA, potentially increasing its use beyond treating benign thyroid conditions.
- Collaboration and Feedback: Ongoing collaboration with clinicians continues to refine RFA technology, with newer generations of electrodes being developed based on direct feedback.
STARmed first introduced thyroid RFA to the world in 2002, kickstarting over two decades of consistent collaboration and innovation. Since then, RF electrodes with thyroid applications have only become sharper, safer, and more precise.
Today, the star RF electrode appears in 80% of all clinical articles about thyroid RFA procedures and efficacy. Likewise, the VIVA II monopolar electrode has become the most advanced electrode in the American market, boasting the first adjustable active tip in the US. Our team of medical innovators continues to address clinician feedback, resulting in the most stable cooling systems, the most comfortable handles, and the sharpest tips in the industry.
That leads many of our trailblazing clinicians to ask, “What’s the next step for thyroid RFA?”
Our dedication to continuous innovation makes us uniquely situated to predict what may be coming next for this efficacious and life-changing technology. In this blog, we’ll speculate on the future of thyroid radiofrequency ablation and RFA technology adoption in the US based on our own observations in the field.
Continue reading to discover what’s to come in the evolving world of radiofrequency ablation for thyroid applications.
Thyroid RFA in America
Per NYU Langone Health, radiofrequency ablation technology received FDA approval for soft tissue masses in February of 2018. It was celebrated as an efficacious and minimally invasive alternative to thyroidectomy for many thyroid concerns, particularly benign nodules. Since then, the use of ultrasound-guided RFA for thyroid applications has increased in popularity across the country.
Over five years later, the earliest adopters remain trailblazers in the field, offering patients non-surgical options with the power to transform their lives, especially if they are not surgical candidates.
The Road to Widespread Adoption
According to a study published in Ultrasonography, diffusion of innovation theory can help us understand the future of thyroid RFA adoption in the United States. The theory suggests that “the less uncertainty that surrounds an innovation and its adoption, the greater chance that it will be diffused into wide-spread use in society.” In other words, the more clinicians pursue RFA training, and the more clinical articles are published about the technology, the more efficiently it will be adopted into widespread use.
Several factors make it more likely to see widespread adoption in the US:
- The reduced cost of RFA compared to surgery for both patients and clinicians.
- Reduced patient recovery time compared to surgery.
- The ability to perform thyroid RFA as an in-office, outpatient procedure without the necessity of anesthesia.
- Reduced risk of hypothyroidism and limited to no need for lifetime medications.
Most of these advantages are well-documented in Asia and Europe, where the adoption of RFA for thyroid applications is more widespread. The more American institutions publish their findings, the more likely the procedure will be to see growth.
Encouragingly, RFA is the most widely studied thermal ablation technique for thyroid nodules, and scientific articles on its use have increased exponentially since its first reference in the year 2000.
Expanding Access to Clinical Training in Thyroid RFA
Likewise, we anticipate that access to robust clinician training resources will be a factor moving forward. Now that COVID-19 restrictions are waning and travel is becoming normalized, opportunities for clinical training will become steadily more accessible. In the meantime, STARmed America is proud to offer STARmed Academy resources both online and in person.
Changing Patient Attitudes Toward Thyroid RFA
Another component of growing this technology is ensuring patients know the procedure is a viable and readily available option. Tools ranging from social media to in-office signage and literature can ensure patients ask the right questions when researching treatment for thyroid nodules. Satisfied patients lead to positive testimonials, ensuring social diffusion as the technology continues to expand.
That’s one reason why STARmed’s STAR Support program offers comprehensive marketing support to clinicians who adopt thyroid radiofrequency ablation in their practices.
The Future Direction of RFA Technology
One of the institutions paving the way for widespread adoption of RFA technology is Columbia University’s Interventional Endocrinology program. Columbia and its director of surgery, Jennifer Kuo, MD, have sponsored new clinical studies that are pushing the field further. Kuo is currently “the only funded researcher in the United States who is evaluating the safety and efficacy of these interventional techniques, in benign nodules, as well as small low-risk thyroid cancers and indeterminate nodules.”
Columbia is responsible for multiple clinical trials that are proving the safety and efficacy of this technology in the literature. These include studies on nodules that have been molecularly profiled benign, as well as low-risk, well-differentiated papillary thyroid carcinomas. They are even making strides in studying the efficacy of using RFA to treat recurrent thyroid cancer.
As larger institutions, such as Columbia, continue to invest in the future of thyroid RFA, we can anticipate the technology to proliferate.
Collaboration Breeds Innovation
Likewise, as innovative medical technology companies continue to collaborate with these pioneering clinicians and institutions, their technology will continue to improve.
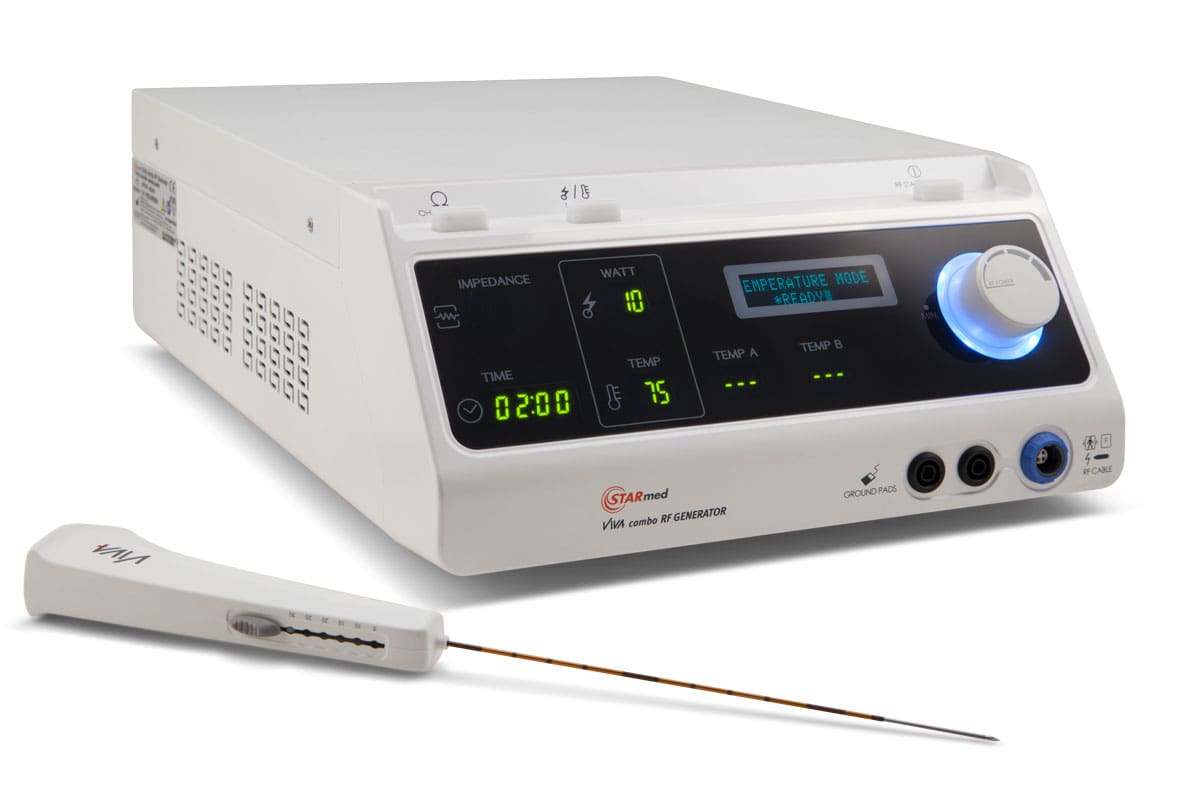
One example of this process in action is the development of the VIVA II monopolar radiofrequency electrode. The VIVA II was the first active tip electrode for thyroid use introduced to the United States. Since its introduction, it has undergone several updates and improvements, all of which were driven by clinician feedback.
In fact, the VIVA electrode you see today is in its third generation, boasting industry-leading features such as:
- Active tip with six different tip lengths for improved versatility, cost reduction, and inventory control.
- Ability to adjust the tip mid-procedure to address multiple nodules of different sizes with efficiency and precision.
- Ultra-stable motor that reduces vibrations, ensuring unmatched control throughout the procedure.
- A lighter, more comfortable handle for enhanced precision and ease of use.
The fourth generation of the VIVA RF electrode and other electrodes depends on the feedback of dedicated clinicians. We look forward to forging new relationships and discovering the future of this technology together.
We encourage interested clinicians to contact a specialist to learn more about purchasing this technology, pursuing training, or marketing their existing services.