Non-scarring. Incision-free. Thyroid preserving
STARmed Thyroid Radiofrequency Ablation for thyroid nodules is a minimally invasive procedure. Our RFA solutions have been helping patients for over two decades. We are proud to have created and developed the first thyroid-specific radiofrequency equipment in the world. As of January 1, 2025, the Centers for Medicare & Medicaid Services(CMS) has established the official CPT® codes for percutaneous radiofrequency ablation of the thyroid to streamline insurance coverage for thyroid RFA.
All STARmed devices are clinically proven and FDA-cleared. For non-functioning benign thyroid nodules, clinical studies have shown 80% volume reduction rate (VRR) at 12 months follow-up and 95% VRR 60 months after the RFA procedure1,2
STARmed first introduced Thyroid Radiofrequency Ablation (RFA) in 2002 in Seoul, South Korea. Today, over 200+ clinical articles worldwide demonstrate the efficacy of this treatment.
STARmed has a complete Thyroid RFA solution for every patient. Our electrodes come in various sizes to address all clinical issues related to the thyroid. That’s why we’re leading the world with our cutting-edge thyroid RFA technology.
Americans have some form of thyroid disease, with the vast majority identified as benign lesions.
thyroid operations are performed each year in the United States.
thyroid resections are performed for benign diseases where patients could benefit from a less invasive option.

The Continuance Mode of the VIVA combo RF Generator produces radiofrequency (RF) output constantly throughout the procedure. Simultaneously, it automatically adjusts the amount of energy applied to the targeted nodule according to real-time impedance feedback. This innovative mechanism ensures the procedure’s safety by minimizing undesired tissue over-ablation.
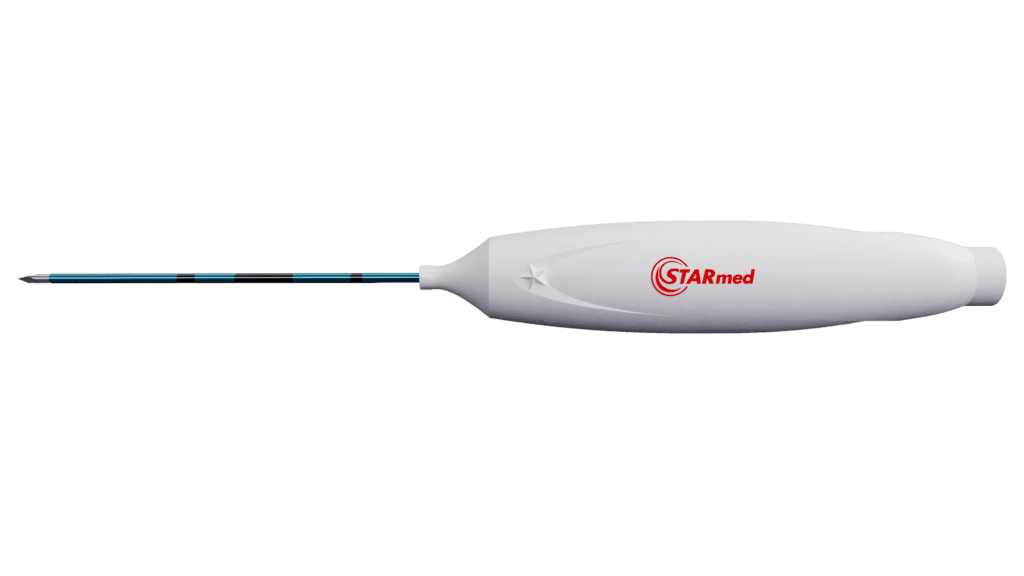
The monopolar star RF Electrode stands out as the first thyroid-dedicated RF electrode in the market. It is uniquely designed with a shorter shaft lengths of 7 or 10 cm. This shorter length is designed to accommodate the gland’s superficial location.
It also has a thinner shaft of 18 or 19 gauge for enhanced energy control. It comes with an array of active tip lengths at 4mm, 5mm, 7mm, and 10mm to cater to different nodule sizes.
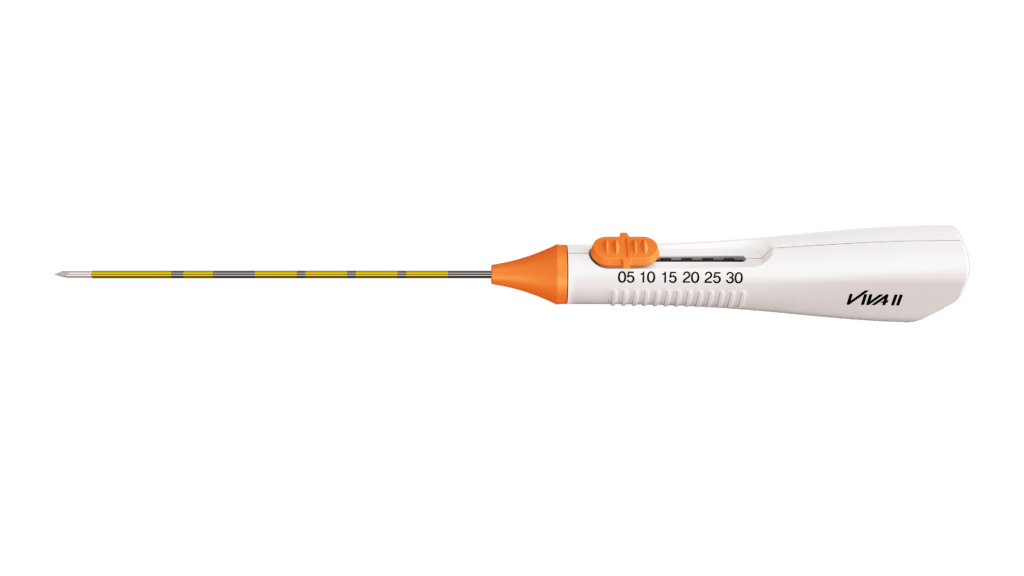
The monopolar VIVA II RF Electrode is the latest innovation in RF ablation. The VIVA Adjustable RF Electrodes are the first of their kind in the world. The VIVA II features an adjustable active tip ranging from 5mm to 30mm. Thus, it can accommodate multiple large nodules of varying sizes with one electrode in one session.
Its latest sharp technology ensures a smooth puncture, while the smaller handle allows for greater precision control. It’s a versatile RFA tool with clinically proven results.
The Continuance Mode and the unique design of the STARmed RF Electrodes enable the Moving-Shot technique. This promotes ablation in units to limit thermal damage to surrounding tissue.
Before STARmed
Thyroid RFA
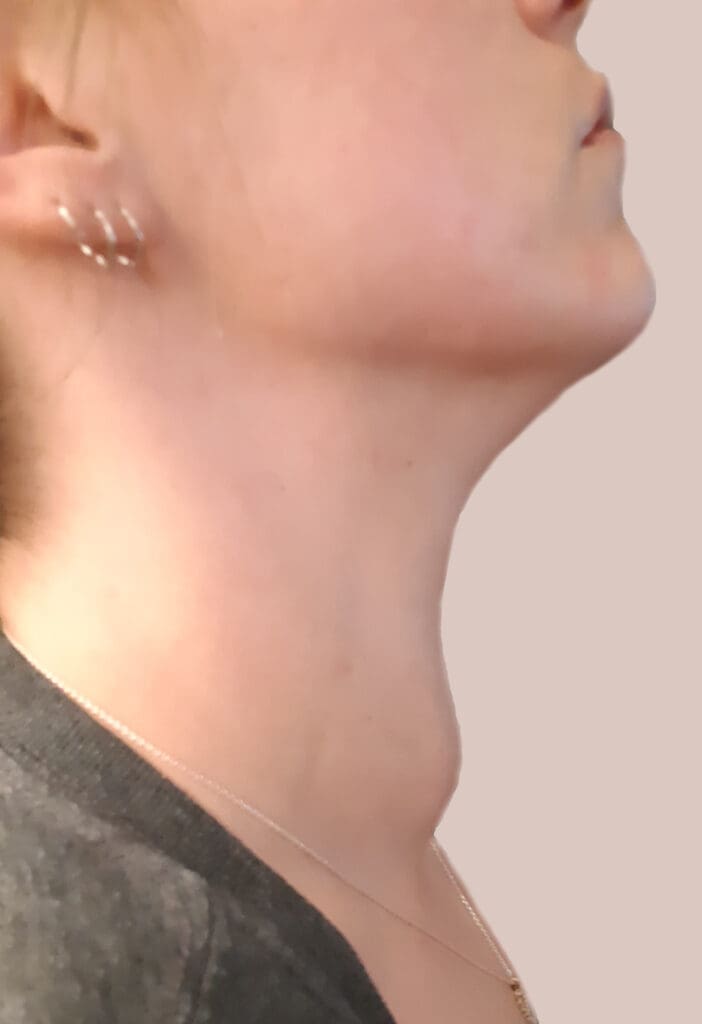
6 months after
STARmed Thyroid RFA

Individual results may vary.
Photo courtesy of Dr. Jung Hwan Baek
STARmed America is committed to refining the customer learning journey. We provide structured and measured procedure training programs for physicians and your care team. Trainings are ongoing and take place in major cities across the country. Commit to improving your practice this year.

The VIVA combo RF System is FDA-cleared. It is intended for use in percutaneous and intraoperative coagulation and ablation of tissue.
Intended Use
The VIVA combo RF System is intended for use in percutaneous and intraoperative coagulation and ablation of tissue.
Star/VIVA RF Electrode is intended for use in percutaneous and intraoperative coagulation and ablation of tissue.
The ELRA Electrode is a radiofrequency (RF) catheter which provides bipolar energy to perform partial or complete ablation of tissue in the pancreatic and biliary tracts.
Quick links
Copyright © 2025 STARmed America. All Rights Reserved. Product names are trademarks or registered trademarks of STARmed America or their respective owners.