STARmed has taken RFA to the next level by introducing ELRATM. Our Endoluminal Radiofrequency Ablation machine & catheter helps patients with unresectable endoluminal hepatic, biliary, or pancreatic lesions. It is a flexible RFA electrode with an automatic Temperature Sensing System. Ultimately, ELRATM offers interventional radiologists a minimally invasive palliative care option to enhance patients’ quality of life.
~20%1
~70%2
<50%3
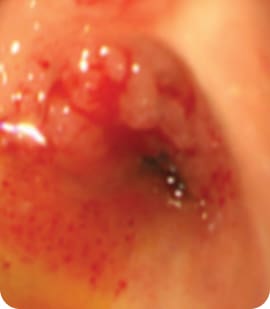
Before RFA
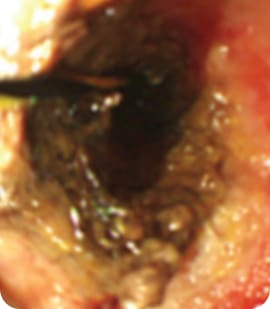
After RFA
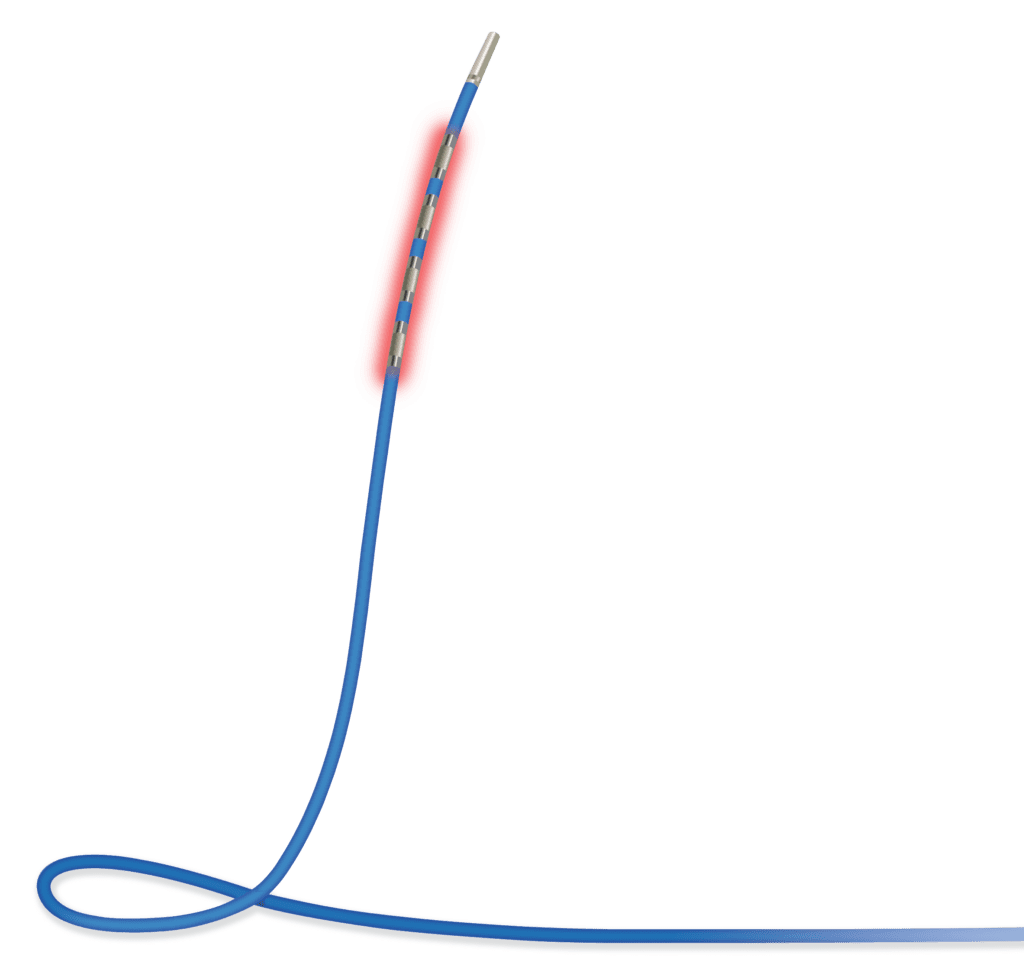
The ELRA Electrode is a radiofrequency (RF) catheter. It provides bipolar energy to perform partial or complete ablation of tissue in the pancreatic and biliary tracts.
Intended Use
The VIVA combo RF System is intended for use in percutaneous and intraoperative coagulation and ablation of tissue.
Star/VIVA RF Electrode is intended for use in percutaneous and intraoperative coagulation and ablation of tissue.
The ELRA Electrode is a radiofrequency (RF) catheter which provides bipolar energy to perform partial or complete ablation of tissue in the pancreatic and biliary tracts.
Quick links
Copyright © 2025 STARmed America. All Rights Reserved. Product names are trademarks or registered trademarks of STARmed America or their respective owners.