products
Endoluminal RF Electrode

The first thyroid-specific radiofrequency ablation (RFA) electrode introduced to the U.S. Referenced in more than 80% thyroid RFA clinical studies, it is engineered for precision and safety in soft tissue ablation.
In 2002, Dr. Jung Hwan Baek performed the world’s first thyroid RFA procedure using STARmed technology, marking the beginning of a new era in minimally invasive thyroid care. Since then, STARmed has continually advanced electrode design and ablation technology, becoming the trusted partner for physicians in more than 100 countries.
1st thyroid RFA with STARmed

First thyroid-specific RFA electrode obtained FDA clearance

1st thyroid RFA electrode launched
Global Leader in Thyroid RFA
Once designed for thyroid RFA, the star RF Electrode now offers a full portfolio for multi-organ use. With the widest range of gauges, shaft lengths, and active tips, it supports precise ablation in thyroid, liver, kidney, bone, and beyond.
18G, 19G, 17G
7, 10, 15, 20, 25, 35 cm
4, 5, 7, 10, 20, 30 mm
18G, 19G, 17G
7, 10, 15, 20, 25, 35 cm
4, 5, 7, 10, 20, 30 mm
Incision-free puncture for smooth entry and reduced trauma.
Enhanced echogenicity ensures precise targeting and clear visibility under ultrasound .
Lightweight and streamlined design allows effortless maneuverability and control.
Circulating saline minimizes tissue charring and ensures predictable zones.
Incision-free puncture for smooth entry and reduced trauma.
Enhanced echogenicity ensures precise targeting and clear visibility under ultrasound .
Lightweight and streamlined design allows effortless maneuverability and control.
Circulating saline minimizes tissue charring and ensures predictable zones.
Clinical studies confirm star RF Electrode’s predictable results and lasting efficacy:
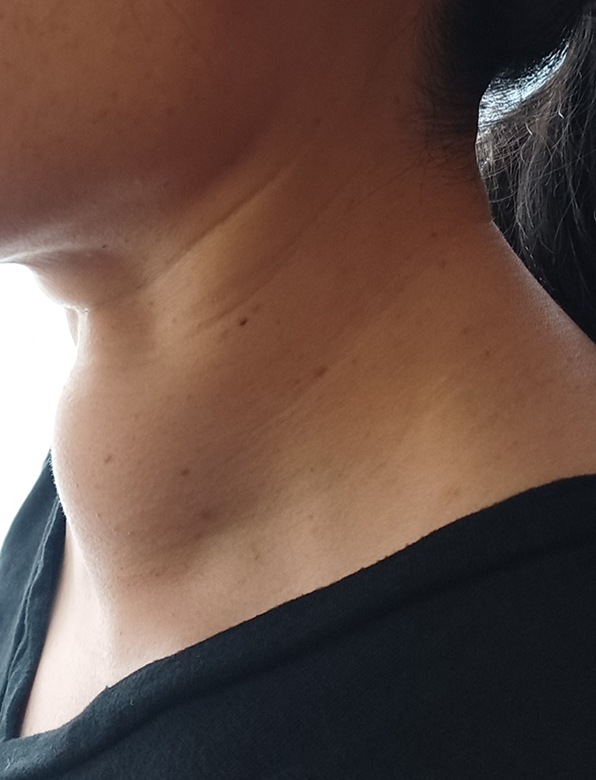
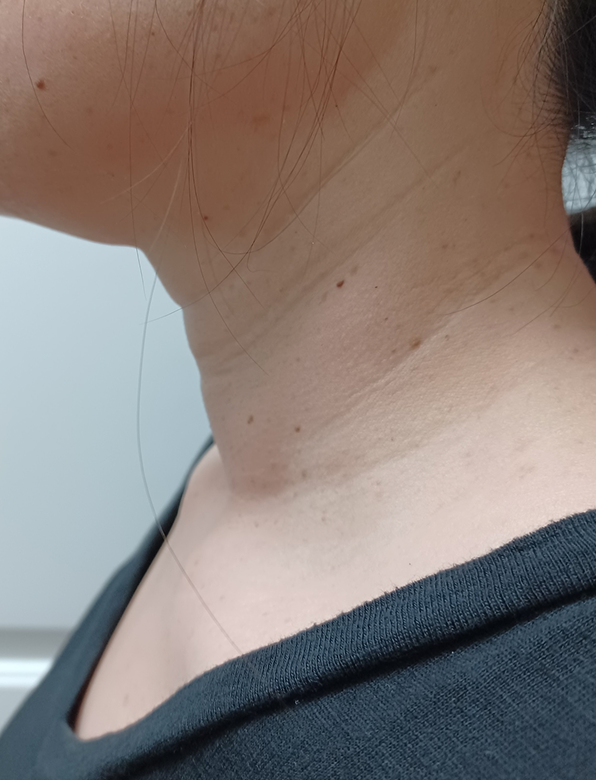


Results may vary. Photos courtesy of Dr. Emad Kandil.
STARmed America goes beyond providing electrodes. We partner with you to ensure success at every step:
Phantom models, peer-to-peer observation, case support.
Coding, prior authorization, appeals guidance.
Referral outreach, patient brochures, social media campaigns.
A monopolar RF electrode is a device used in thermal ablation procedures. It delivers focused energy to a targeted area to treat conditions in tissues such as the thyroid, liver, or kidney. STARmed’s monopolar RF electrode is designed for precision, efficiency, and safety.
In monopolar systems, a grounding pad (also known as a dispersive electrode) is essential. It’s placed on the patient’s body—typically on the thigh or back—to safely return the electrical current from the electrode, completing the circuit and ensuring the energy is dispersed without harming surrounding tissue. This setup enhances both the safety and effectiveness of the ablation procedure.
The STARmed RF ablation electrode uses radiofrequency energy to create controlled thermal lesions in tissue. Its internal cooling system enhances the ablation margin while reducing the risk of tissue charring.
Patients typically see nodule shrinkage within 4–6 weeks, with continued improvement at 6–12 months
Yes. As of January 1, 2025, percutaneous thyroid RFA has Category I CPT®️ codes (60660 & 60661), allowing physicians to bill for the procedure using standard CPT coding. Coverage varies by payer and region, and most insurers require preauthorization. STARmed America provides reimbursement support, including coding guidance, preauthorization assistance, and appeal resources.
Experience the electrode trusted worldwide for thyroid and other soft tissue ablation. Supported by STAR Support, your team is backed from the 1st case to the 500th.
See how safe and effective STARmed’s industry-leading equipment is in practice.

By submitting this form, you consent to receive marketing emails from STARmed America at the email provided. You can unsubscribe at any time. Please see our privacy policy for more information.