Non-surgical. Incision-free. Uterine preserving.
The radiofrequency ablation procedure (RFA) for fibroids has been around since the 1990s. The technique uses heat to achieve contact coagulative necrosis of the fibroids.
At STARmed, we pioneered radiofrequency ablation for uterine fibroids with transvaginal ultrasound guidance.
Our devices seamlessly integrate with almost all available ultrasound systems. Transvaginal ultrasound-guided radiofrequency ablation of uterine fibroids eliminates the need for incisions. Fibroid RFA is a simpler procedure for physicians. Most crucially, it’s a truly minimally invasive solution that relieves patient symptoms while preserving their uterus.
Fibroid RFA is an efficient, minimally invasive procedure performed in an outpatient setting under local anesthesia. Most patients return to normal activities in a single day. Patients experience peace of mind knowing the radiofrequency ablation procedure has a low complication rate.
women in the U.S. are currently diagnosed with fibroids.
hysterectomies are performed for benign indications annually in the U.S
of women want to avoid invasive surgeries and long recovery times.
STARmed’s uterine fibroid radiofrequency ablation procedure utilizes real-time transvaginal ultrasound guidance to accurately locate fibroids. This facilitates the insertion of the star RF Electrode into the targeted fibroid. With transvaginalultrasound, your patients will experience precise ablation initiation and effective fibroid treatment in a more comfortable and less invasive manner. STARmed’s fibroid RFA techniques can also be used for hysteroscopic and laparoscopic radiofrequency ablation of uterine fibroids depending on the locations of the fibroids.
The star RF Electrode is designed to work seamlessly with nearly all ultrasound systems. Likewise, it is fully compatible with your existing transvaginal ultrasound probes. The electrode is inserted through the ultrasound probe guide during the procedure. This ensures continuous in-plane visualization for optimal accuracy.
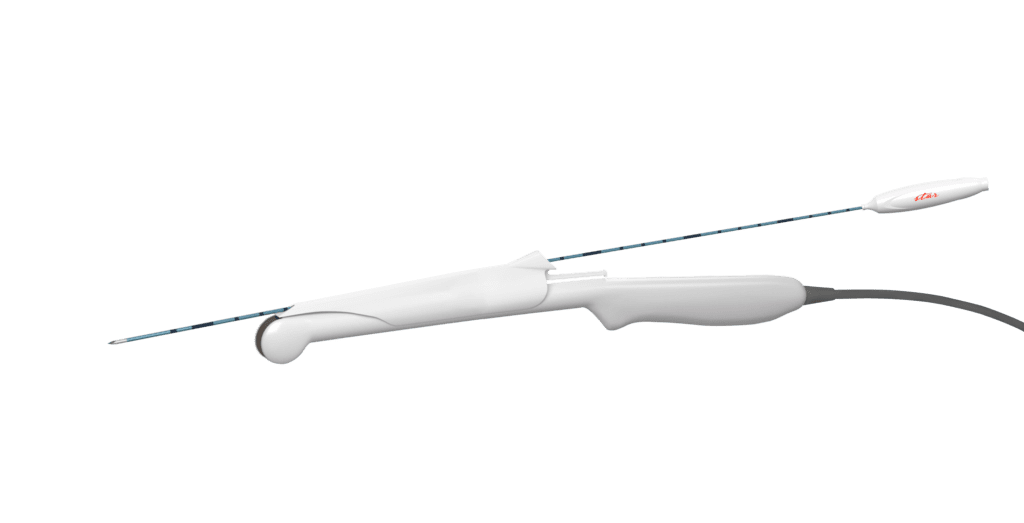

The star RF Electrode comes with 2 shaft lengths, 25 cm, and 35 cm. It also has 3 different active tip lengths, 1.0 cm, 2.0 cm, and 3.0 cm, to suit different clinical needs.
STARmed’s cool-tip technology is specifically designed to promote the STAR Stepping Target Ablation in Real-time technique. Partnering with the internal cooling system and the real-time impedance feedback via the VIVA combo RF System, STARmed Fibroid RFA maximizes ablation zones without damaging the surrounding structures.
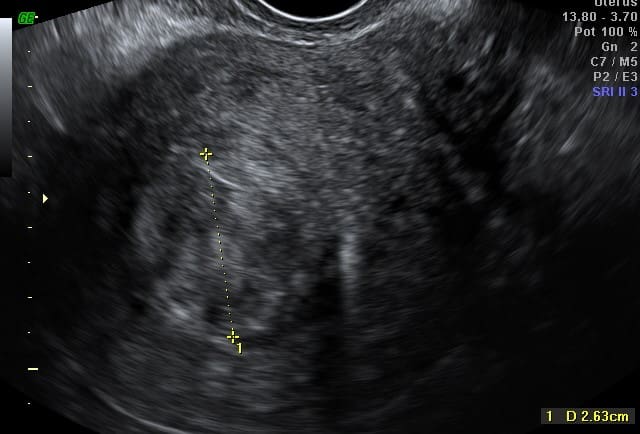
Before
STARmed Fibroid RFA
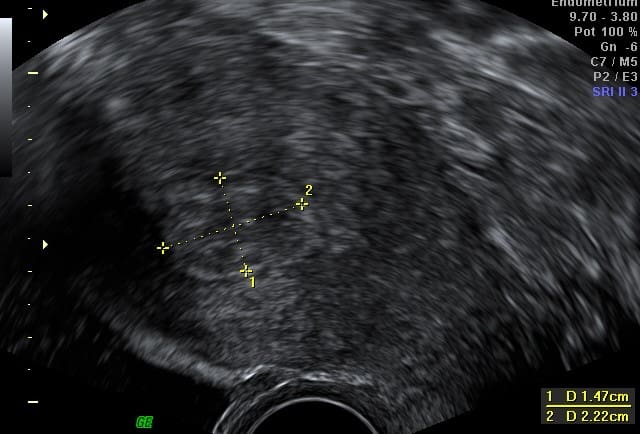
6 months after
STARmed Fibroid RFA
The VIVA combo RF System is FDA-cleared. It is intended for use in percutaneous and intraoperative coagulation and ablation of tissue.