products
Endoluminal RF Electrode
The first monopolar electrode with an adjustable active tip. VIVA II delivers high-energy ablation for larger lesions and controlled treatment at the margins — all with one device
With its adjustable tip length, VIVA II eliminates the need to stock multiple electrodes for different cases. This adaptability makes it possible to perform both precise ablations near critical structures and larger central ablations in a single session.
Incision-free puncture for smooth entry and reduced trauma
Enhanced shaft metal material improves ultrasound visibility for safe lesion targeting
Lightweight handle with upgraded slider and clear level markings for easy control
Circulating saline minimizes tissue charring and supports consistent ablation zones.
All with one electrode, reducing setup time and inventory needs
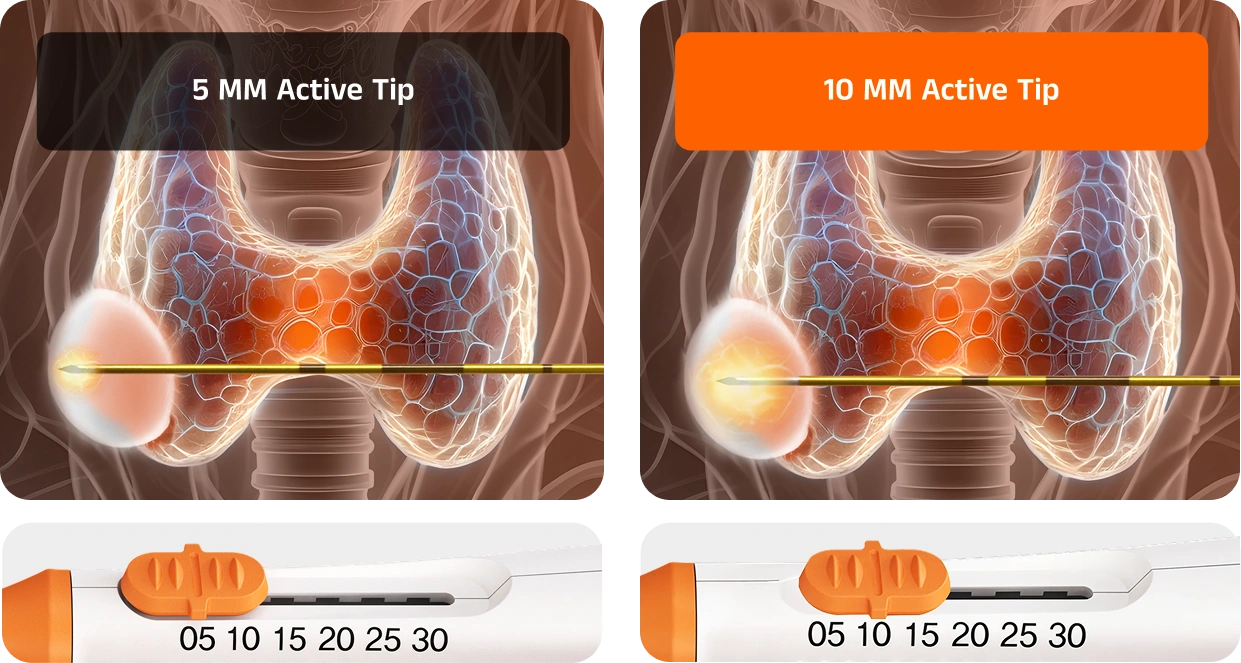
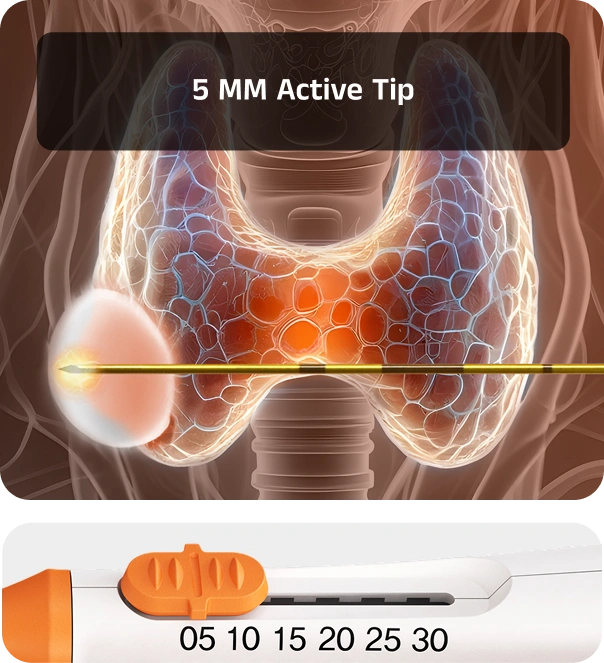
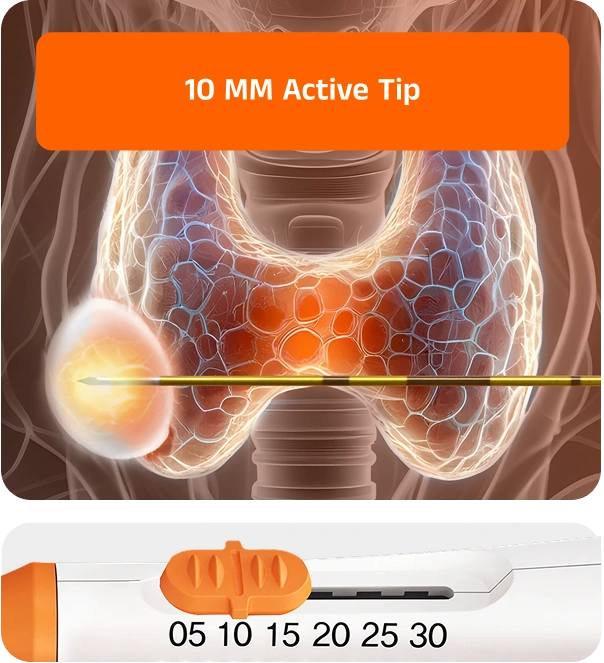
Safely achieve both large central ablation and fine peripheral ablation to reduce marginal regrowth risk, all with one electrode.
Multiple studies have validated STARmed’s adjustable and monopolar RF electrodes for thyroid RFA, demonstrating both safety and long-term efficacy:
Feasibility & safety of adjustable electrodes for benign thyroid nodules¹
Shorter ablation times and efficient workflow compared to fixed electrodes²
Reduced marginal regrowth with small-tip peripheral ablation³
Global adoption across multiple organ systems supported by procedural and technique reviews⁴
Feasibility & safety of adjustable electrodes for benign thyroid nodules¹
Shorter ablation times and efficient workflow compared to fixed electrodes²
Reduced marginal regrowth with small-tip peripheral ablation³
Global adoption across multiple organ systems supported by procedural and technique reviews⁴
From training and live case support to reimbursement guidance and marketing tools, STAR Support ensures you succeed beyond the procedure room.

Yes. The VIVA II RFA needle electrode features an adjustable active tip with five length options, allowing clinicians to toggle mid-procedure for both large central and small peripheral ablations without switching devices.
Yes. The VIVA II monopolar electrode is the first radiofrequency ablation needle with an adjustable active tip technology cleared for use in the United States. This FDA clearance ensures the device meets strict safety and efficacy standards for clinical practice.
Yes. Monopolar needle electrodes such as the VIVA II are designed for safe, repeated use in clinical ablation procedures when utilized according to their IFU. The adjustable ablation electrode design also minimizes the need for multiple devices, reducing patient risk and improving procedural efficiency.
Simplify your workflow and expand your treatment options. With VIVA II, consistent results come from one electrode, not many.
See how safe and effective STARmed’s industry-leading equipment is in practice.

By submitting this form, you consent to receive marketing emails from STARmed America at the email provided. You can unsubscribe at any time. Please see our privacy policy for more information.